n47Brn019icePhysical Properties:

Image: stockstotrade.com
- Appearance: Colorless, transparent solid when pure
- Density: 0.917 g/cm³ (water: 1 g/cm³)
- Melting point: 0 °C (32 °F)
- Boiling point: 100 °C (212 °F)
Chemical Properties:
- Molecular formula: H₂O
- Molecular weight: 18.02 g/mol
- Polarity: Highly polar molecule
- Solubility: Forms solutions with many polar solvents
- Reacts with: Strong acids, bases, and certain metals
Other Properties:
- Can float on water: Due to its lower density than liquid water
- Forms various crystalline structures: Hexagonal structure is the most common
- Congealing point: The temperature at which liquid water starts to freeze (slightly below 0 °C)
- Sublimation: Can transition directly from a solid to a gas without passing through a liquid phase
- Latent heat of fusion: The energy required to melt ice (334 kJ/kg)
- Latent heat of vaporization: The energy required to vaporize ice (2256 kJ/kg)
Uses:
- Refrigerant: Used to preserve food and drinks
- Transportation: Used as a preservative during the transportation of perishable goods
- Construction: Used in both solid and liquid forms (as a coolant)
- Medical: Used in cooling pads, surgery, and cryotherapy
- Beauty: Used as an ingredient in skincare products
- Geological: Forms glaciers, ice caps, and polar ice
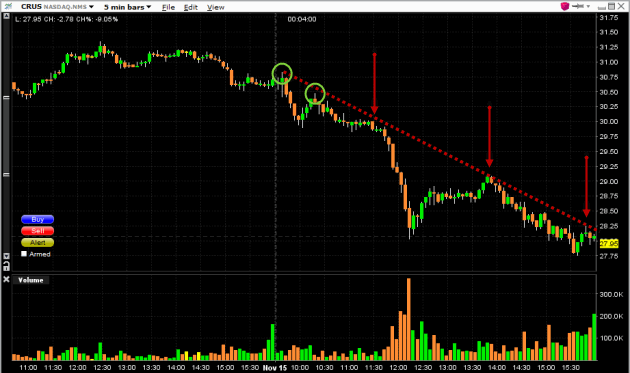
Image: www.tradinggraphs.com
Stock Options Day Trading

Image: purepowerpicks.com